Search
- Page Path
- HOME > Search
Original Article
- Miscellaneous
- AM1638, a GPR40-Full Agonist, Inhibited Palmitate- Induced ROS Production and Endoplasmic Reticulum Stress, Enhancing HUVEC Viability in an NRF2-Dependent Manner
- Hwan-Jin Hwang, Joo Won Kim, SukHwan Yun, Min Jeong Park, Eyun Song, Sooyeon Jang, Ahreum Jang, Kyung Mook Choi, Sei Hyun Baik, Hye Jin Yoo
- Endocrinol Metab. 2023;38(6):760-769. Published online November 2, 2023
- DOI: https://doi.org/10.3803/EnM.2023.1774
- 1,222 View
- 85 Download
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub - Background
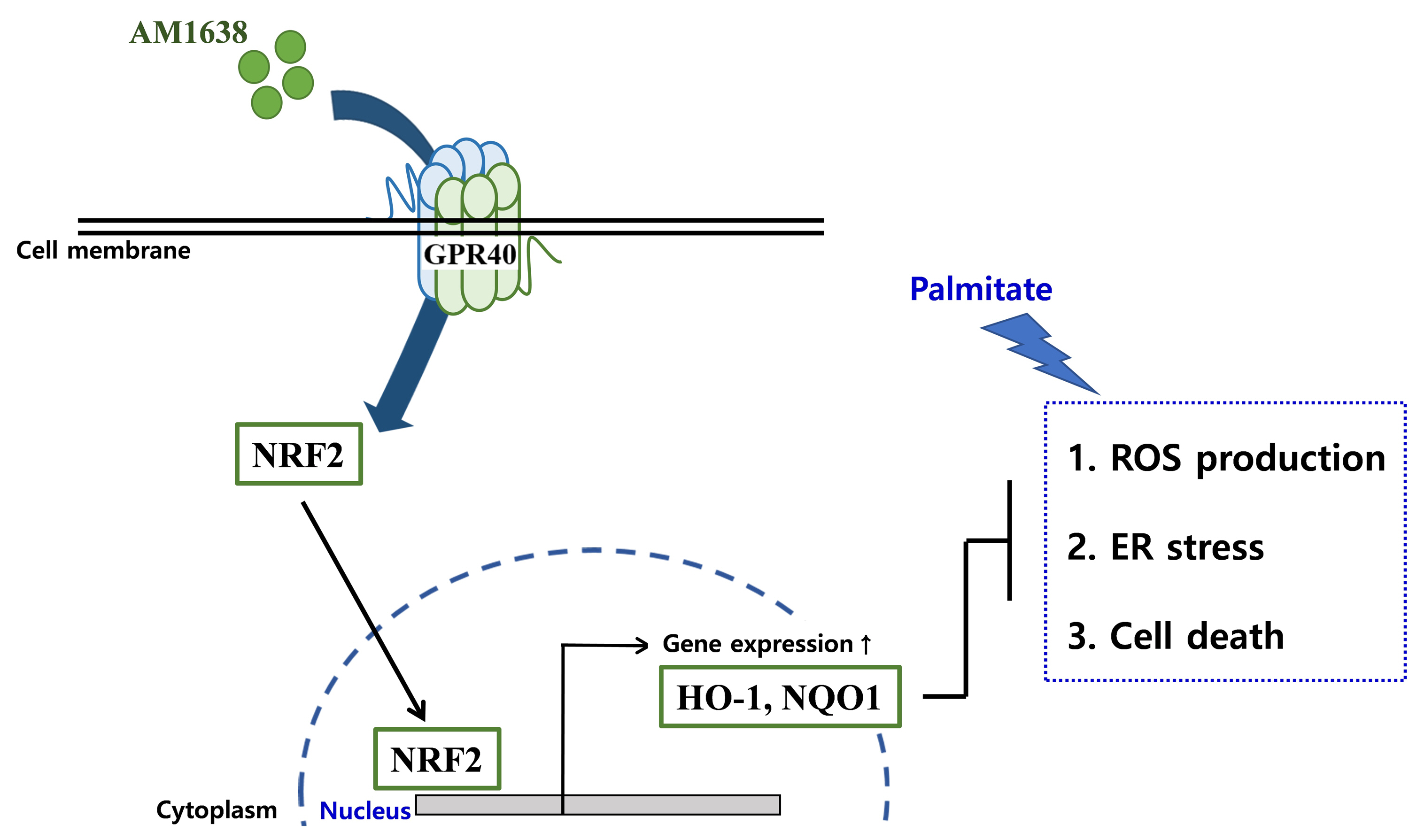
G protein-coupled receptor 40 (GPR40) is a key molecule in diabetes and fatty liver, but its role in endothelial dysfunction remains unclear. Our objective in this study was to determine whether GPR40 agonists protect endothelial cells against palmitatemediated oxidative stress.
Methods
Human umbilical vein endothelial cells (HUVECs) were used to investigate effects of various GPR40 agonists on vascular endothelium.
Results
In HUVECs, AM1638, a GPR40-full agonist, enhanced nuclear factor erythroid 2–related factor 2 (NRF2) translocation to the nucleus and heme oxygenase-1 (HO-1) expression, which blocked palmitate-induced superoxide production. Those antioxidant effects were not detected after treatment with LY2922470 or TAK875, GPR40-partial agonists, suggesting that GPR40 regulates reactive oxygen species (ROS) removal in a ligand-dependent manner. We also found that palmitate-induced CCAAT/enhancer‐binding protein homologous protein expression; X-box binding protein-1 splicing, nuclear condensation, and fragmentation; and caspase-3 cleavage were all blocked in an NRF2-dependent manner after AM1638 treatment. Both LY2922470 and TAK875 also improved cell viability independent of the NRF2/ROS pathway by reducing palmitate-mediated endoplasmic reticulum stress and nuclear damage. GPR40 agonists thus have beneficial effects against palmitate in HUVECs. In particular, AM1638 reduced palmitate-induced superoxide production and cytotoxicity in an NRF2/HO-1 dependent manner.
Conclusion
GPR40 could be developed as a good therapeutic target to prevent or treat cardiovascular diseases such as atherosclerosis.

Brief Report
- Diabetes, Obesity and Metabolism
- Sestrin2 Regulates Beneficial β3-Adrenergic Receptor-Mediated Effects Observed in Inguinal White Adipose Tissue and Soleus Muscle
- Min Jeong Park, Joo Won Kim, Eun Roh, Kyung Mook Choi, Sei Hyun Baik, Hwan-Jin Hwang, Hye Jin Yoo
- Endocrinol Metab. 2022;37(3):552-557. Published online June 29, 2022
- DOI: https://doi.org/10.3803/EnM.2022.1421
- 2,653 View
- 107 Download
- 2 Web of Science
- 2 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
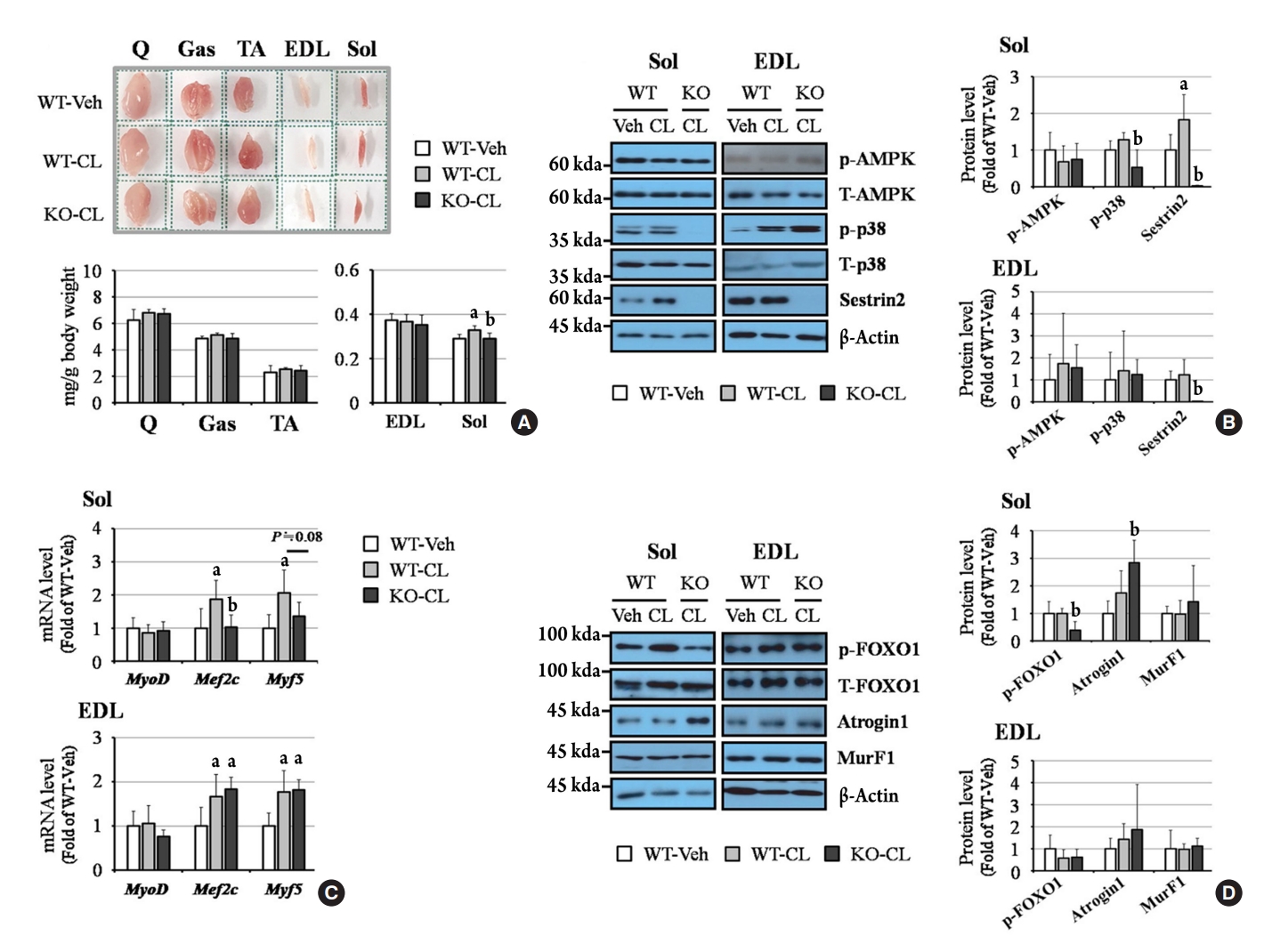
ePub - Sestrin2, a well-known adenosine monophosphate-activated protein kinase (AMPK) regulator, plays a protective role against metabolic stress. The β3-adrenergic receptor (β3AR) induces fat browning and inhibits muscle atrophy in an AMPK-dependent manner. However, no prior research has examined the relationship of sestrin2 with β3AR in body composition changes. In this study, CL 316,243 (CL), a β3AR agonist, was administered to wild-type and sestrin2-knockout (KO) mice for 2 weeks, and fat and muscle tissues were harvested. CL induced AMPK phosphorylation, expression of brown-fat markers, and mitochondrial biogenesis, which resulted in the reduction of lipid droplet size in inguinal white adipose tissue (iWAT). These effects were not observed in sestrin2-KO mice. In CL-treated soleus muscle, sestrin2-KO was related to decreased myogenic gene expression and increased levels of muscle atrophy-related molecules. Our results suggest that sestrin2 is associated with beneficial β3AR-mediated changes in body composition, especially in iWAT and in the soleus.
-
Citations
Citations to this article as recorded by- Sestrin2 levels in patients with anxiety and depression myocardial infarction was up-regulated and suppressed inflammation and ferroptosis by LKB1-mediated AMPK activation
Yufeng Qian, Lian Chen, Beibei Gao, Xianhua Ye
Clinical and Experimental Hypertension.2023;[Epub] CrossRef - Sestrin2 in diabetes and diabetic complications
Xiaodan Zhang, Zirui Luo, Jiahong Li, Yaxuan Lin, Yu Li, Wangen Li
Frontiers in Endocrinology.2023;[Epub] CrossRef
- Sestrin2 levels in patients with anxiety and depression myocardial infarction was up-regulated and suppressed inflammation and ferroptosis by LKB1-mediated AMPK activation

Original Article
- Thyroid
Big Data Articles (National Health Insurance Service Database) - Risk of Diabetes in Patients with Long-Standing Graves’ Disease: A Longitudinal Study
- Eyun Song, Min Ji Koo, Eunjin Noh, Soon Young Hwang, Min Jeong Park, Jung A Kim, Eun Roh, Kyung Mook Choi, Sei Hyun Baik, Geum Joon Cho, Hye Jin Yoo
- Endocrinol Metab. 2021;36(6):1277-1286. Published online December 16, 2021
- DOI: https://doi.org/10.3803/EnM.2021.1251
- 5,200 View
- 181 Download
- 9 Web of Science
- 10 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
The detrimental effects of excessive thyroid hormone on glucose metabolism have been widely investigated. However, the risk of diabetes in patients with long-standing hyperthyroidism, especially according to treatment modality, remains uncertain, with few longitudinal studies.
Methods
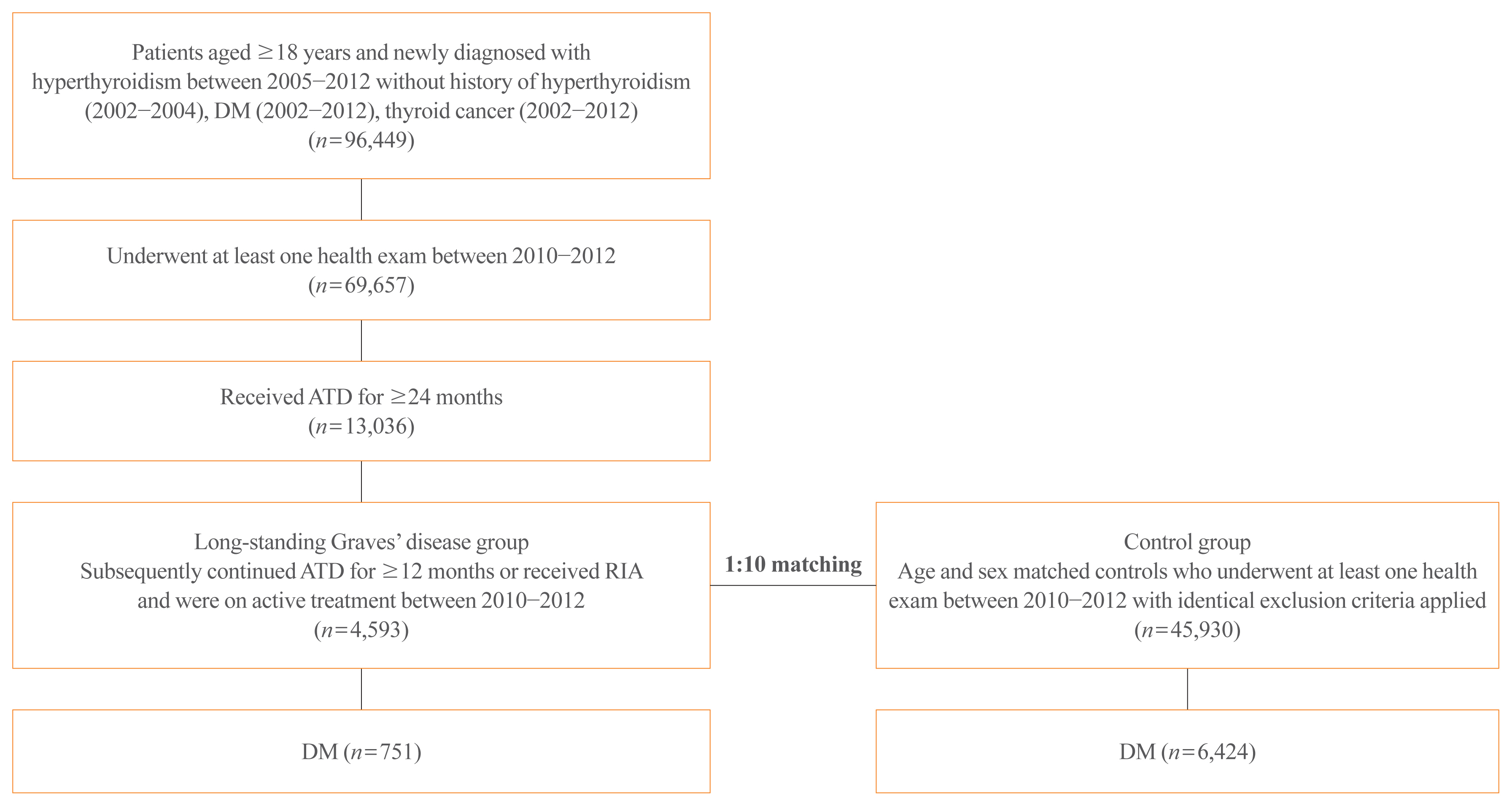
The risk of diabetes in patients with Graves’ disease treated with antithyroid drugs (ATDs) for longer than the conventional duration (≥2 years) was compared with that in age-and sex-matched controls. The risk was further compared according to subsequent treatment modalities after a 24-month course of ATD: continuation of ATD (ATD group) vs. radioactive iodine ablation (RIA) group.
Results
A total of 4,593 patients were included. Diabetes was diagnosed in 751 (16.3%) patients over a follow-up of 7.3 years. The hazard ratio (HR) for diabetes, after adjusting for various known risk factors, was 1.18 (95% confidence interval [CI], 1.10 to 1.28) in patients with hyperthyroidism. Among the treatment modality groups, the RIA group (n=102) had a higher risk of diabetes than the ATD group (n=4,491) with HR of 1.56 (95% CI, 1.01 to 2.42). Further, the risk of diabetes increased with an increase in the ATD treatment duration (P for trend=0.019).
Conclusion
The risk of diabetes was significantly higher in patients with long-standing Graves’ disease than in the general population, especially in patients who underwent RIA and prolonged ATD treatment. Special attention to hyperglycemia during follow-up along with effective control of hyperthyroidism may be necessary to reduce the risk of diabetes in these patients. -
Citations
Citations to this article as recorded by- Safety of non-standard regimen of systemic steroid therapy in patients with Graves’ orbitopathy: a single-centre experience
Nadia Sawicka-Gutaj, Dawid Gruszczyński, Natalia Zawalna, Kacper Nijakowski, Agnieszka Skiba, Mateusz Pochylski, Jerzy Sowiński, Marek Ruchała
Pharmacological Reports.2024; 76(1): 185. CrossRef - Increased risk of diabetes mellitus and hyperlipidemia in patients with differentiated thyroid cancer
Hwa Young Ahn, Jooyoung Lee, Jinmo Kang, Eun Kyung Lee
European Journal of Endocrinology.2024; 190(3): 248. CrossRef - Prevalencia de diabetes en personas con disfunción tiroidea
Juan J. Díez, Pedro Iglesias
Medicina Clínica.2023; 160(8): 333. CrossRef - Control of Thyroid Dysfunction in Spanish Population Registered in
the Primary Care Clinical Database: An Analysis of the Proportion of Patients
with Thyrotropin Values Outside the Reference Range
Juan J. Díez, Pedro Iglesias
Hormone and Metabolic Research.2023; 55(03): 184. CrossRef - Prevalence of thyroid dysfunction and its relationship to income level and employment status: a nationwide population-based study in Spain
Juan J. Díez, Pedro Iglesias
Hormones.2023; 22(2): 243. CrossRef - Prevalence of diabetes in people with thyroid dysfunction
Juan J. Díez, Pedro Iglesias
Medicina Clínica (English Edition).2023; 160(8): 333. CrossRef - Diabetes Mellitus Secondary to Endocrine Diseases: An Update of Diagnostic and Treatment Particularities
Mihaela Simona Popoviciu, Lorena Paduraru, Raluca Marinela Nutas, Alexandra Maria Ujoc, Galal Yahya, Kamel Metwally, Simona Cavalu
International Journal of Molecular Sciences.2023; 24(16): 12676. CrossRef - Thyroid Eye Disease and Its Association With Diabetes Mellitus: A Major Review
Roshmi Gupta, Pramila Kalra, Lakshmi B. Ramamurthy, Suryasnata Rath
Ophthalmic Plastic & Reconstructive Surgery.2023; 39(6S): S51. CrossRef - Metabolite Changes during the Transition from Hyperthyroidism to Euthyroidism in Patients with Graves’ Disease
Ho Yeop Lee, Byeong Chang Sim, Ha Thi Nga, Ji Sun Moon, Jingwen Tian, Nguyen Thi Linh, Sang Hyeon Ju, Dong Wook Choi, Daiki Setoyama, Hyon-Seung Yi
Endocrinology and Metabolism.2022; 37(6): 891. CrossRef - Diabetes and Hyperthyroidism: Is There a Causal Link?
Sang Yong Kim
Endocrinology and Metabolism.2021; 36(6): 1175. CrossRef
- Safety of non-standard regimen of systemic steroid therapy in patients with Graves’ orbitopathy: a single-centre experience


 KES
KES

 First
First Prev
Prev



